News concerning the safety of food seems to be everywhere these days. On a daily basis there’s a story about a salmonella outbreak or a company initiating a product recall due to possible contamination. Why is this the case?
If you visit most food businesses, whether it’s a restaurant, grocery store, manufacturer or foodservice operator, chances are you’ll see the same thing: Employees using pen and paper checklists, forms and log books to manage their food safety operations.
The recent E. coli outbreak traced to Chipotle Mexican Grill infected more than 50 people and led the company to shut down several restaurants. The outbreak was also a PR disaster for the company and damaged its reputation as a reliable provider of safe meals. Chipotle lost out on potential revenue and probably spent a good amount of money on hiring outside food safety consultants to examine its safety standards.
Since starting the business, Chipotle has remained focused on a core mission: Make great-tasting food, and more recently, food that is not modified with GMOs. While its goal has not changed, running a food company is vastly different today than in the past.
Modern Food Safety Isn’t So Modern
For one thing, there is a lot more paper to manage in today’s world. Between time and temperature controls, HACCP and HARPC requirements, and a whole host of industry certifications and brand standards, food businesses implement several safety processes. Even with advancements in technology, food safety operations are often run manually and therefore are error-prone.
In the early 1990s, food companies could handle the volume of paperwork themselves. Today, they’re swamped. Visit a food business, and you’ll see the same thing everywhere: Stacks of documents that need to be typed up and sent to food agencies. As one quality assurance manager recently stated, “We can barely keep track of them all.”
Surrounded by stacks of paper in their office, quality assurance (QA) managers explain that much of the pileup is due to more rules and regulations related to food safety. Food companies must comply with a growing number of local, state and federal laws that regulate food safety. The focus of recent laws such as FSMA is toward prevention of foodborne illness, placing even more emphasis on internal audits and recordkeeping. In addition to these laws, food companies must compete with the wealth of information available to customers about how their food safety operations work. Especially in the realm of social media, as Taco Bell has learned, one photo of an employee playing with food can lead to a PR nightmare.
A Day in the Life of a QA Manager
Complying with food safety laws often falls on a company’s QA manager who supervises food safety. She walks through the facility several times a day with clipboard in hand, reviewing a list of safety and quality measures.
The QA manager will then manually key this data into a spreadsheet, create reports, and file the results with industry partners and government regulators. These seemingly routine and time-consuming compliance tasks matter. Failing to comply with the appropriate laws can lead to costly penalties, permitting delays, loss of business from industry partners (such as retailers with strict requirements), and even legal action.
The legal requirements are often complex, overlapping, and they change every couple of years. The laws are designed, of course, to ensure that food preparation and delivery is safe, thereby protecting consumers. But an expanding body of regulations and fear of litigation have increased the time, cost and stress that play into compliance.

Improve Food Safety with Technology
So how can companies improve their food safety operations? By using food safety technology, particularly mobile software tools, to improve their processes. Since food safety operations are still manual, they tend to be hard to standardize and difficult to track—especially at larger companies where employees are working in multiple shifts across dozens of locations. Mobile food safety software offers several major benefits:
- No More Pen and Paper. Replacing paper-and-pencil clipboards with digital tools saves time and money. Digital audits and task-lists can be logged and tracked, ensuring that staff are performing tasks in real-time. Digital entries are more accountable; managers can confirm when and where tasks where conducted and completed (including requiring photos to be taken). And digital clipboards can be loaded with reference materials like images and training videos, which helps staff learn best practices and prepare for real inspections by government agencies.
- Quality and Safety Checklists. Instead of letting employees complete tasks ad hoc and make notes on clipboards and log books, companies can use quality and safety checklists to ensure that key tasks are standardized across the organization. For example, data can be collected to show that a company is always forgetting to label produce with an expiration date. Digital food safety and quality checklists that are loaded on smartphones or tablets makes it easier to ensure that all employees are following brand standards and best practices.
- Automated Reports. Instead of sifting through binders filled with audit logs, food safety software captures and stores data in a structured format, making it easy to search and analyze. Why waste hours at the end of every week or month sifting through binders full of paper, when software lets you generate insights with the click of a button?
- Real-Time, Centralized Management. Food companies often have multiple locations in which employees are conducting food safety operations in their own way. For companies that have multiple locations, mobile software being used by employees at each location can help corporate managers track performance by location, provide critical alerts, and give employees real-time feedback to help standardize food safety operations.
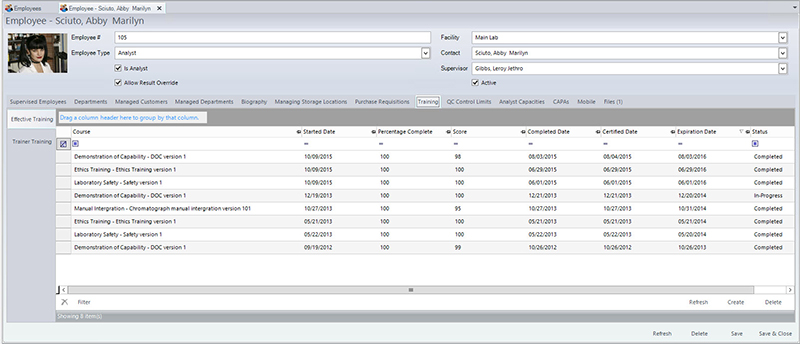
Here’s an example of a QA manager running a food safety audit using mobile software. During a random spot check, the manager shows up on the line with a smartphone in hand. As she walks around, she pulls up a food safety application and answers a series of pre-set multiple choices questions that cover key criteria, dictates comments into the device using the built-in voice recognition, and takes high-resolution color photos of several problematic issues. If a QA manager is unsure about food safety requirements, she can use her mobile device to quickly pull up a reference document (or even the official code citation) from state, FDA, USDA or other agencies.
After running a digital audit with food safety software, the QA manager can immediately print or e-mail a report that shows all of the items out of compliance, creating actionable intelligence for her team. The QA manager can then share this with line workers during their weekly team meeting, which help to train staff on best practices in food safety.
The data the QA manager collected through her mobile device is immediately stored in the cloud. From there it can be easily accessed by a colleague (i.e., her manager at corporate headquarters) at any time. Over time, the data from each of these spot checks is stored in a central database that a manager can analyze, looking for trends in performance, issues that keep arising, or locations that may need extra training and attention. Mobile software makes it easier to generate insights that can drive major improvements in an organization’s safety and performance.
By using software to help manage food safety audits, logs and line checks, businesses can save time and money on compliance, train staff on best practices, and most importantly, keep customers safe and satisfied.
Today, food safety technology, especially mobile software, should be a critical part of any modern food company’s operations. Mobile audit and task-management software allows QA managers to streamline and standardize quality and safety operations across large teams and multiple locations, helping save valuable time and money. Whether you’re a mobile food vendor or a large-scale food processor, modern software tools can help food businesses of all sizes effectively manage time-consuming tasks around food safety and compliance, from digitizing audit logs for analysis to created automated filings for supply chain partners.