Monitoring for veterinary drug residues is conducted to ensure food safety and compliance with approved veterinary medicine practices. Veterinary drugs are used in animal husbandry for a variety of reasons, including as a curative/preventive of disease in herd and flock, to improve meat quality, and to promote growth. The chemical classes of drugs that may be used are broad, but major classes include antibiotics, anti-parasitics, and hormones. While risk-modifiers are used to minimize risk for consumption, residues from these drugs, their breakdown metabolites, or associated impurities of the drug may persist in animal tissue, necessitating the requirement that contaminant testing be undertaken.
In the United States, trace analysis of contaminants in food products began in the early 1970s following amendments to the Federal Food, Drug, and Cosmetic Act (FFDCA) in 1968. Worldwide, the regulatory requirements for contaminants in food have seen significant tightening due to a number of high-profile contamination crises and increased trade of food across country borders. From the technology standpoint, lower detection limits have been made possible by improvement of the detection capabilities of the analytical methods and instruments. Some of the most stringent requirements for contaminants in food are found in the European Union, where the levels of contamination should be below Minimum Residue Limits (MRLs), whereas in the United States, such limits are called U.S. tolerances.

When analyzing for drug residues, the choice of tissue has historically been the liver and kidney tissues, as these organs serve to remove the contaminants from the body and, as a result, the concentration of contaminants there is higher and easier to detect. Muscle tissue now often is added to the target list, as its contamination would have a direct impact on consumers.
With regards to veterinary drugs testing, one can distinguish between screening methods and confirmatory methods. The former should be fast and high-throughput and used to detect the presence of an analyte. The confirmatory methods should be able to provide confirmation of an analyte’s identity and quantitation at the levels of interest. Microbiological methods were popular for screening of antimicrobial drugs since these drugs inhibit growth of microorganisms, but suffer from a lack of specificity since not all microorganisms are equally sensitive to all antibiotics. Rapid screening methods include immunoassay-based testing kits, which are specific, fast, and can include multiple antibiotic classes in one test. Confirmatory methods typically include chemical analysis techniques with LC-MS detection, which provides the best ionization for most classes of veterinary drugs, along with better selectivity for focused analysis and lower detection limits. LC-MS can provide specific analysis of compounds from multiple classes in the same run through either targeted MS/MS or non-targeted analysis of unknowns through high mass resolution methods. The speed of LC-MS analysis has improved with the introduction of ultra-high pressure liquid chromatography-MS (UHPLC-MS) instruments. In the last few years, UHPLC-MS methods simultaneously serve as screening and confirmation methods for multiple classes, so called “multi-residue methods”. Some of these methods use MS/MS detectors and some use high-resolution mass spectrometers utilizing time-of-flight and ion trap detectors. These methods now can provide fast turn-around time and better accuracy in comparison to microbiological methods. They may be preferentially used by testing laboratories that are equipped and capable of utilizing the latest MS instrument technologies.
The 4th Annual Food Labs conference provides practical solutions and best practices on running, managing and equipping a food lab. | March 7–8, 2016, Atlanta, GA | LEARN MOREAll mass spectrometry methods that strive to perform simultaneous analysis of multiple veterinary drug classes are prone to the same drawbacks. Due to the differences in the analytes’ polarity, acidity and hydrophobicity, the quantitative extraction of analytes from tissue samples could be difficult. Ideally, the sample preparation methods should be compatible for compounds with varying physico-chemical properties but still provide selective separation from the matrix components to avoid occurrence of matrix effects during quantitation. The co-extracted matrix impurities are undesirable since they can affect the ionization of targeted analytes and result in under- or over-estimation of their concentration (ion suppression or enhancement). Due to the difficulty in designing a method that works for a wide variety of analytes, cleanup is often omitted for multi-class multi-analytes methods, and the stable isotope internal standards are used to correct for ionization effects during quantitation. However, omitting the sample cleanup could lead to other methodology problems.
As noted in the veterinary drug analysis session during the 2015 AOAC Annual meeting, sample cleanliness can result not only in matrix effects and impact quantitation, but it can also have an effect on the mass accuracy when high-resolution mass spectrometry is used and, therefore, can affect the identification of the analytes and lead to false negatives.
The most often used methodologies for sample cleanup during analysis of veterinary drugs in tissues is solid-phase extraction (SPE), both in cartridge and dispersive formats. C18 SPE proved to be a very versatile sorbent that often resulted in the best cleanup and best precision of analysis, closely followed by polymeric sorbents when applied to multi-class LC-MS analysis.
Aminoglycosides Antibiotics
Aminoglycosides is one class of veterinary antibiotics that is hard to include into multi-class methods. The aminoglycoside structures include connected modified sugars with different number of substituents including hydroxy- and amino-groups. The higher degree of polarity for aminoglycosides contributes to their solubility properties: these compounds are freely soluble in water and to some extent are soluble in lower alcohols, but are not soluble in common organic solvents and have solubility issues in solvent-water mixtures with high organic contents. Therefore, the normal extraction conditions that include organic solvents and are frequently applied to most other classes of veterinary drugs do not work well for aminoglycosides. A separate method is often used to extract and analyze these antibiotics.
Most often aminoglycosides are detected by mass spectrometry through the formation of positive ions during electrospray ionization. The LC separation of aminoglycosides could be done by either a reversed-phase (RP) method with ion-pair mobile phase additive to insure the retention of compounds or by HILIC chromatography. We have investigated both methods and looked at the sensitivity for detection of these compounds. The use of ion-pair is most often presented as a disadvantage, as it can reduce the analyte signal through the decrease of ionization efficiency and fouling the LC-MS instrument. While the use of ion-pair in our study decreased the ionization for some of the lighter compounds in this class (streptomycin, puromycin), ionization efficiency increased for the heavier mass compounds (gentamycin, neomycin). RP chromatography resulted in improved separation of the analytes compared to HILIC. LC-MS fouling from the use of HFBA was not observed in our investigation that spanned the course of a couple of years. In the HILIC mode with use of formic acid as a mobile phase additive, the detection of neomycin was problematic due to very low sensitivity. It was as low as one seventh of the sensitivity obtained by RP method.
The instrument response for aminoglycosides also depends on sample extraction and cleanup and the accompanying matrix ionization effects. The extraction from animal tissues has been traditionally done using the McIlvaine buffer that includes 2% Tricloroacetic acid (TCA) to precipitate proteins and release any bound analytes and 0.4 mM EDTA to prevent the binding of the analytes to cations and/or glass. Then the extract undergoes cleanup steps using SPE. The SPE sorbent most often used is a cation exchange phase, as the aminoglycosides have ionizable amino-groups and can be retained from the extract through ion-exchange interactions. Another option for the SPE cleanup became recently available—molecularly imprinted polymeric (MIP) SPE. MIPs, which are sometimes called “chemical antibodies”, mimic the performance of immunoaffinity sorbents. MIPs have binding sites that conform to the shape and functionality of a specific compound or a compound class. Strong binding of the analyte to the MIP makes it possible to perform intensive SPE washes that lead to very clean samples. Unlike immunoaffinity sorbents, MIPs are compatible with organic solvents and strong acids and bases.
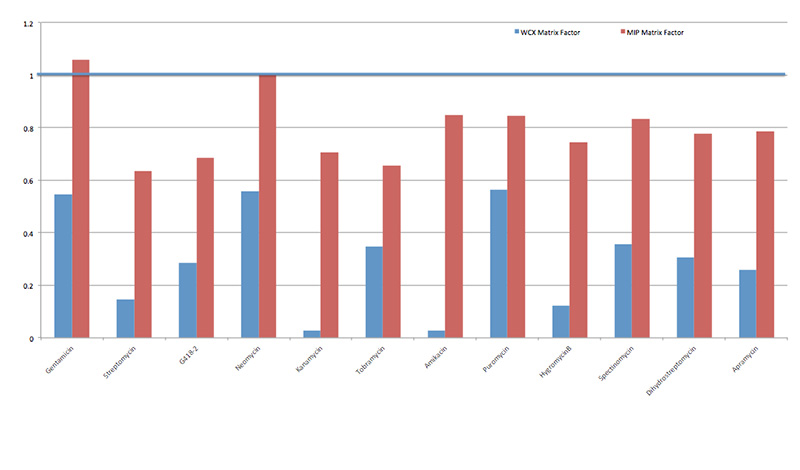
 We have tested the MIP SPE versus the traditional weak cation exchange (WCX) SPE cleanup for aminoglycosides spiked into pork tissue. The resulting ionization effects were compared as an indication of samples cleanliness. The quantitation in both cases was done using matrix-matched calibration curves and in both cases the recoveries for most of the ten tested aminoglycosides were above 70% (with exception of spectinomycin at 33% in case of WCX cleanup and tobramycin at 55% in case of MIP cleanup). For the two cleanup methods, there was a significant difference in matrix effects. In Figure 1, matrix factors close to 1.0 indicate little to no matrix influence for analyte detection: the ionization of the analyte in mass spectrometer is not influenced by co-extracted matrix impurities and quantitation values are not skewed. Values for matrix factors that are significantly greater than 1.0 suggest matrix enhancement for the analyte and values less than 1.0 are considered to be the result of matrix suppression. Significant matrix suppression was observed for all analytes when WCX SPE was used for cleanup. The ion suppression effect was significantly less for samples cleaned using MIP SPE. In addition, we observed significant time savings when using the MIP SPE cleanup method, as it did not require sample evaporation after using water-containing elution solvent.
We have tested the MIP SPE versus the traditional weak cation exchange (WCX) SPE cleanup for aminoglycosides spiked into pork tissue. The resulting ionization effects were compared as an indication of samples cleanliness. The quantitation in both cases was done using matrix-matched calibration curves and in both cases the recoveries for most of the ten tested aminoglycosides were above 70% (with exception of spectinomycin at 33% in case of WCX cleanup and tobramycin at 55% in case of MIP cleanup). For the two cleanup methods, there was a significant difference in matrix effects. In Figure 1, matrix factors close to 1.0 indicate little to no matrix influence for analyte detection: the ionization of the analyte in mass spectrometer is not influenced by co-extracted matrix impurities and quantitation values are not skewed. Values for matrix factors that are significantly greater than 1.0 suggest matrix enhancement for the analyte and values less than 1.0 are considered to be the result of matrix suppression. Significant matrix suppression was observed for all analytes when WCX SPE was used for cleanup. The ion suppression effect was significantly less for samples cleaned using MIP SPE. In addition, we observed significant time savings when using the MIP SPE cleanup method, as it did not require sample evaporation after using water-containing elution solvent.
Conclusions
While improvement in the laboratory instrumentation allows the simultaneous and fast analysis of multiple contaminants, sample preparation remains important for reliable identification of contaminants in screening methods and error-free quantitation in confirmatory methods. Both the extraction and sample cleanup methods can contribute to accurate multi-class methods analyzing wide variety of veterinary drugs. New and upcoming technologies such as molecularly-imprinted polymers could be used for more targeted analysis of specific classes of analytes via instrumental methods.







