Compassion is at the core of food safety, and it is a trait that shines through for anyone who has had the opportunity to meet Melody Ge. As the Director of Food Safety & Quality Assurance (FSQA) at StarKist, which produces nearly 50% of canned food goods on the market, and founder of Women in Food Safety, Ge has devoted her career to helping others—both by protecting consumers and by nurturing young professionals.
We spoke with Ge to learn more about her background, her career and what drives her success as a food safety leader.
What led you to a career in food safety?
Ge: My mom worked for food safety labs, and I knew that she was doing something good that was helping society. After graduating from University of Maryland with a Food Science, Technology and Nutrition degree, I started my job with Beyond Meat in R&D and food safety & quality. During my time there, I was on a business trip with one of my grad school classmates who accidently had a serious shrimp allergic reaction at a restaurant where we ate together during the trip. Even though he emphasized that he is allergic to shrimp to the waiter. This was over 10 years ago, and it still gives me goose pumps. I am always a person willing to help, and to see him go through that was a traumatic experience.
At that moment, I understood firsthand the critical role that food safety plays in society. That experience combined with my own work experience made me want to focus on food safety, and I found my passion. I started to focus my career path on safety and quality by working for GFSI CPOs, EU retailers, manufacturers, and other stakeholders.
What are some of the biggest challenges you’ve faced working in food safety and quality assurance?
Ge: I always say that FSQA professionals are heroes because they take a lot of what they do to heart. They put a lot of responsibility on their own shoulders to protect consumers. Often, FSQA professionals are seen as the police of operations. So, communication is one of the challenges: how we can translate the technical knowledge and share the sense of urgency to other department stakeholders within the company so we can achieve FSQA together within the business?
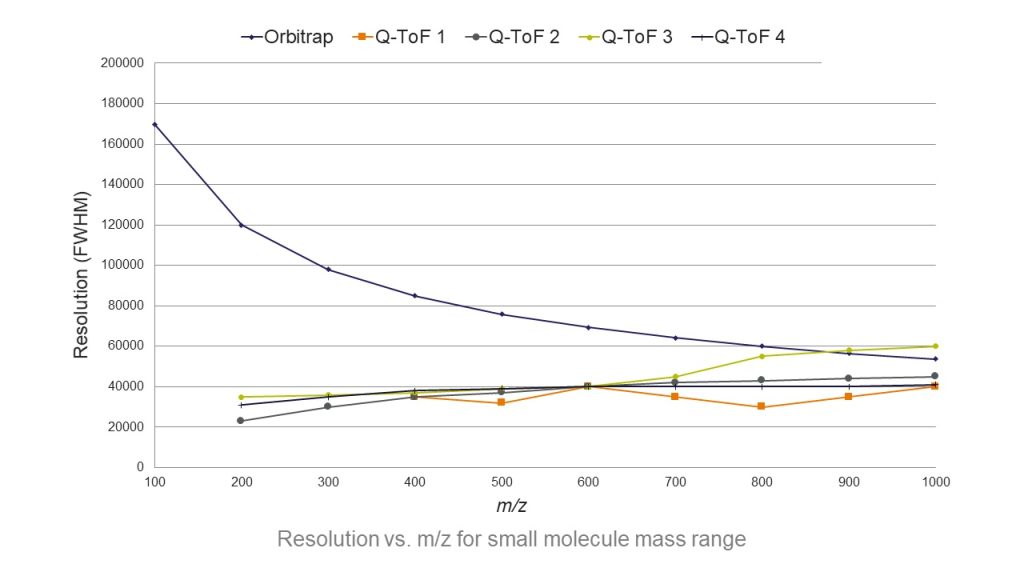
The other challenge is on the technical side. We deal with an evolving environment. For example, what we knew about listeria 10 years ago is different than what we know today. The regulations are always changing. Hence, keep ourselves updated and keep learning are crucial.
Do you have any tips or strategies on how to do that in the midst of doing your day-to-day job?
Ge: My advice would be to use the pieces or fragments of time. You don’t have to devote two hours of your day to learn a new policy, for example, the new FDA traceability rules. Sometimes, while I’m sitting down having my coffee, I am scanning the news, and that’s learning. When I’m having lunch, I try to look at some webinar recordings, and that’s learning. When you have small pieces of time throughout the day, you actually can learn quite amount of new information. Subscribe to the industry publications like Food Safety Tech and Food Quality, and learn from everyone around you. I learn from my team and my coworkers. I also send them to webinars and then we learn from each other.
You mentioned that a lot of people view FSQA as the police of the company. How do you overcome that?
Ge: Being an influencer, proactive communicator and trusted member of the team are keys to success. I find a way to communicate all these important aspects to the team at Starkist. I do feel lucky that at Starkist I am working with people who are aware of food safety and quality constantly. And now with social media and the direct face to consumers it offers, people overall are more aware of food safety and quality. There is a fundamental basic knowledge out there.
I try to use the audience’s language, whether its senior management or production employees. I also stay connected with the line people. Every time I’m in the plant, I walk with them and talk with them. I make them aware that I’m not picking on them; it’s about the products that get produced and consumed. And I am still practicing this every day to be better.
You are also the founder of Women in Food Safety. When did that group start, and what led you to put that together?
Ge: The group started in January 2020 with the intention of helping the younger generations. The initial idea was to provide a resource and a platform for students and industry, and this evolved after I met my committee members. Now we have two missions: First to pipeline the younger generation and second to help bridge the gap between academia and industry.
We have five focuses:
- Diversity in Culture. We really focus on supporting people who are coming from different cultures to help them adapt within their companies.
- Adventure Starts. This is for the students and early first and second year professionals in the industry
- Leadership. Believe it or not, there were a lot of females stuck in at the manager level for over eight years, and then it’s very hard to move up. This focus is to help them climb that ladder to eventually become an executive in the industry.
- Boots on the Ground. One of the challenges in food safety is how we work with the line people at the plants to communicate food safety and how to adapt our working style in the manufacturer environment.
- Work and Life Balance. This is not just for women who are having children; it’s also about how to take your breaks in life, enjoy your downtime and your family, and still stay up to date and come back to the industry as a new leader or professional at any time you are ready again.
How can people get involved in Women in Food Safety?
Ge: We have a LinkedIn group. You do need to be approved to get in just to keep the group focused on the mission and the industry needs, and keep it from being diluted into a commercial group. The group now has around 900 members. With our two—almost three—years partnership with Food Safety Tech, we have more and more influence. We now hold in-person events at the Food Safety Consortium and also at IAFP with the students. We also have a website, and it’s free to subscribe.
If you could turn the clock back to when you were just starting in the industry, what would you tell your younger self and would you have made different decisions?
Ge: I would say, “You are on the right path! Don’t let the difficulties and challenges happening in your career distract you or change you. You know who you are and you know what you’re doing.”
I don’t regret any of my decisions because they all made me the Melody I am today. In some cases, I chose to leave a very good boss for a better career, which was very hard for me. Those decisions and challenges still make me sad till today but I don’t regret those moves.
When people ask me, “How did you get such a wide variety of experience?” It is because I stepped out of my comfort zone, even though it was scary. I made decisions for myself that long-term I knew were going to help my career.
What advice would you offer professionals who are just starting their careers in food safety?
Ge: Try different things and say yes. Just say yes! Every time I get the question, “Can you do this?” I say, “Yes!” and then I figure it out. Don’t hesitate when there are new opportunities, and learn from anything you do at the moment. When I first started, I worked for three years in customer service. I answered emails and phone calls from suppliers who had technical questions. Was it a really fun job? Maybe not. But it helped me so much even up to today when I’m implementing any GFSI CPOs, I remember the details of the clauses. So, enjoy what you do—that is the foundation of doing a job well. Be patient, and keep in mind that nothing you do will be wasted. It’s all part of your own puzzle, and those pieces will eventually all come together.
What’s your opinion on mentors and mentorship?
Ge: One thing about mentoring I do want to share is that it is not a matter of saying, “I need a mentor so I’m going to go out and find myself one!” Mentoring is a concept. It’s a chemistry that naturally happens between two people learning from each other. You know this person will help you; or maybe it’s their style that influences you, and you don’t feel awkward to be vulnerable in front of them. There are many professionals in the industry who are my mentors—sometimes they might not know it. I learn from them and translate what I learn in a way that I can maintain based on my personality, so it’s sustainable.
What’s the main driver that keeps you in food safety?
Ge: Every day is a different day. I am not a person who likes doing things according to a preset list, meaning when you walk into the office you know exactly what you’re going to do that day. I enjoy investigating and identifying problems and finding solutions. That’s what keeps me in FSQA.
Another thing is this is a very friendly industry. I really like the people who work in food safety and quality. We are open to each other. We share best practices and knowledge. We ask questions and we share knowledge. We are like friends and family.
What are some of your hobbies or interests outside of work?
Ge: I try to learn something new every year. Each January, I set a personal goal for myself for the year. For example, in the past, I have learned photography, flamenco, surfing and so on. Some I maintained, and some I don’t because I don’t like them after I tried. Last year, I started learning Korean. I am a scuba diver and a yoga instructor. I try to explore new things each year. I am not an expert on those different things, but they expose me to new ideas, which keeps me energized.