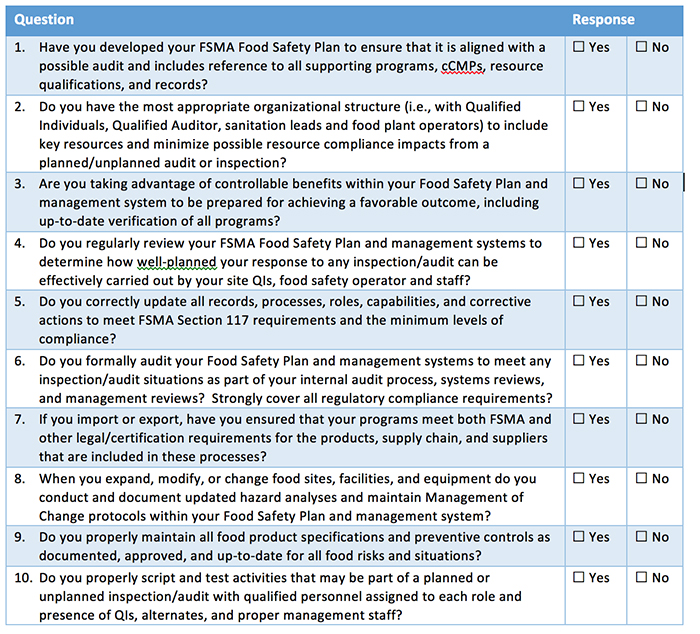
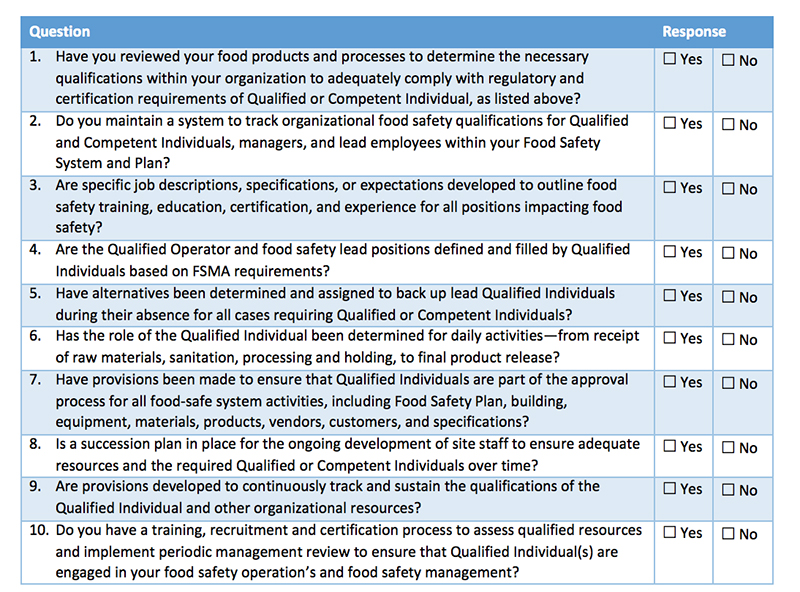
September 19, 2016 is a date that many of you probably had circled on your calendars. It marked the first date in which many food processing companies had to be in compliance with the FSMA preventive controls final rule.
It’s okay if you’re still revising your food safety plan. The regulations are so sweeping that some companies are still struggling to figure out if their plans are in compliance. At the heart of this law is a change in the philosophy of how we deal with contamination. Now, the focus is on preventing contamination rather than responding to it after it occurs.
This proactive approach to safety must be kept in mind when discussing how food safety plan requirements have changed. For many food manufacturing facilities, it means a change from HACCP to HARPC.
Hazard Analysis and Critical Control Points, or HACCP, should be more familiar to you. First developed in the late 1950s and early 1960s to provide safe food for astronauts in the U.S. space program, HACCP became the global standard for food safety in the 1980s, as large, multinational companies sought to ensure that their supply chains were safe.
HACCP evolved over the years into an effective, efficient and comprehensive food safety management approach. The system addresses food safety through the analysis and control of biological, chemical and physical hazards from raw material production, procurement and handling, to manufacturing, distribution and consumption of the finished product.
The seven principles of HACCP include:
- Conduct a hazard analysis
- Identify critical control points
- Set critical limits
- Establish monitoring actions
- Determine corrective actions
- Develop verification procedures
- Institute a record-keeping system
How are HACCP and HARPC different?
Following the passage of FSMA, the FDA instituted a new set of food safety standards, known as Hazard Analysis and Risk Based Preventive Controls (HARPC).
HARPC shouldn’t be seen as a replacement of HACCP standards. Rather, it’s an evolution of them. The following are some key changes.
You Must Anticipate Potential Hazards. One of the big changes in moving to HARPC standards is that your food safety plan must identify any and all reasonably foreseeable food safety hazards and include risk-based preventive controls for them. This moves beyond HACCP’s critical control points and asks that food processors look at how to minimize risk from the second food enters their facility to the second it ships out.
This includes naturally occurring hazards as well as hazards that can be intentionally or unintentionally introduced to the facility. The potential hazards that have expanded under HARPC include:
- Biological, chemical, physical and radiological hazards
- Natural toxins, pesticides, drug residues, decomposition, parasites, allergens and unapproved food and color additives
- Naturally occurring hazards or unintentionally introduced hazards
- Intentionally introduced hazards (including acts of terrorism)
You should review the potential hazards—both seen and unseen—that could impact your facility to determine the risks that you should analyze for your plan.
HARPC Applies to Almost All Food Processing Facilities. The HACCP standards generally did not apply to all food processors. HARPC, however, covers many more U.S. processors. There are six major exceptions, however.
- Food companies under the exclusive jurisdiction of the USDA
- Companies subject to the FDA’s new Standards for Produce Safety authorities
- Facilities that are subject to and comply with FDA’s seafood and juice HACCP regulations
- Low-acid and acidified canned food processors
- Companies defined as “small” or “very small” businesses
- Companies with a previous three-year average product value of less than $500,000
Do these changes mean that your existing food safety plan needs to be scrapped? Not at all. An existing HACCP plan can be modified with the help of a Preventive Control Qualified Individual (another new requirement) to comply with HARPC guidelines. This person needs to be intimately familiar with potential hazards and the risk-based preventive controls for them.
This may sound daunting at first, but moving to HARPC from HACCP will be an easier shift than starting from scratch. The key adjustments that you would need to focus on include identifying risk-based preventive controls for the hazards previously mentioned. Just remember, these hazards should be expanded to include both naturally occurring and unintentionally introduced hazards.
How Does Integrated Pest Management Fit into a Food Safety Plan?
Much like HARPC, Integrated Pest Management (IPM) focuses on being proactive. It emphasizes prevention, focusing on facility maintenance and sanitation, before considering chemical options for pest management.
An IPM plan is benchmarked with regular monitoring and analysis of effectiveness. This may seem cumbersome, but one shouldn’t overlook the value of documentation as a management tool. Collecting data and putting it in context with detailed analysis can be an effective way to prioritize your pest control efforts.
Detailed analysis accounts for things such as normal seasonal cycles, deficiencies in maintenance, exclusion, sanitation and harborages, just to name a few. This analysis can also help improve pest control efforts by prioritizing areas needing attention, especially when your staff is limited by time or resources.
Integrating IPM into your HARPC plan should include analyzing the risks of what could encourage pests to enter your facility, such as doors left open or incoming product shipments. Consider your pest control provider an expert source in how to assess all risks associated with pests and how to establish preventive controls for them.
Despite preventative efforts, unexpected pests will be inevitable. More emphasis will be placed on establishing action thresholds for different pests. This can be a problematic topic, because there are not scientific or broadly accepted threshold values for food processing pests.
Every facility, and often zones within facilities, will likely be different. Identify logical zones—ingredients, processing, packaging and warehousing—and sensible threshold values for each key pest in these zones. Furthermore, establish what the appropriate response should be at certain thresholds. The escalating responses to different levels of pest activity often include things such as automatic authority for certain limited types of pesticide application, more intensive monitoring and inspection, and, of course, higher management notifications, which might lead to more extensive measures.
IPM plans should be reviewed on an annual basis to ensure your program remains as effective as possible. Written food safety plans that follow the HARPC approach and comply with the FSMA rule should be reanalyzed whenever there is a significant change at the facility that might increase a known hazard or introduce a new one. Review the plan at least every three years, if no significant changes occur.
Even if your facility’s deadline for compliance with HARPC standards is a year or two away, now is the time to take a look at your plan and make sure you’re in compliance.