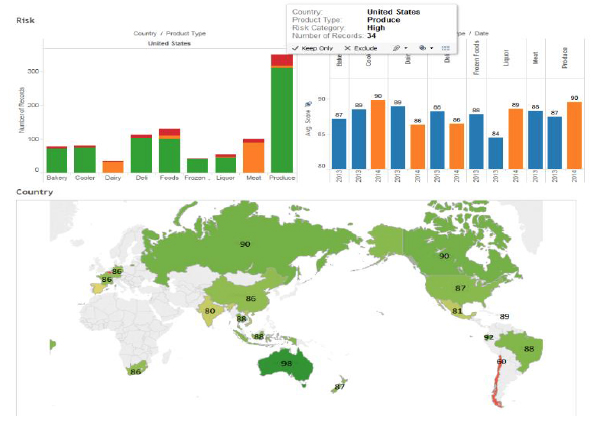
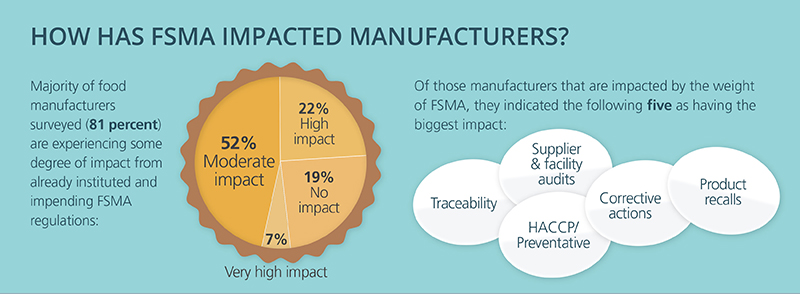
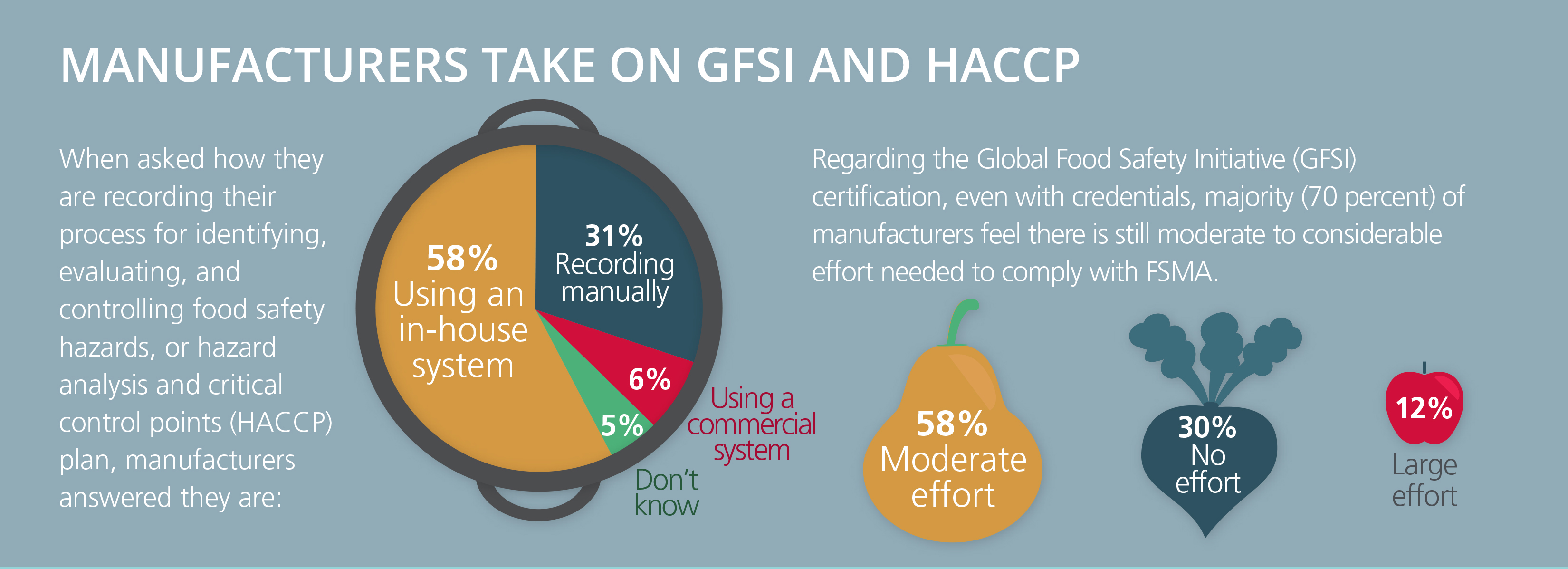
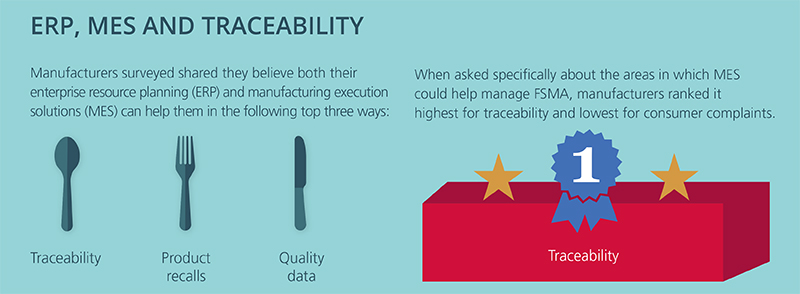
The FSMA Third Party Accreditation (TPA) final rule was published in the Federal Register in final form on November 27, 2015. Although TPA is not limited to imported food, its primary use will most likely be for food imports. TPA offers foreign food facilities and food importers a way to show FDA that the items coming to the United States meet federal food safety requirements.
An acceptable audit by a certified auditor is the only way an importer can take advantage of another FDA program, the Voluntary Qualified Importer Program (VQIP), which offers expedited review and entry of food. If FDA deems it necessary, the agency can also require certified audits for the import of specific foods.
The TPA process requires a number of administrative steps by FDA and non-FDA entities before the first third-party inspection is made. The four major steps are:
- FDA is responsible for officially recognizing accreditation bodies.
- An officially recognized accreditation body will accredit third-party certification bodies.
- The accredited third-party certification body will certify third-party auditors.
- The certified auditors will conduct consultative and regulatory audits of food facilities.
If FDA does not find an applicant that it can officially recognize as an accreditation body within two years, it may directly accredit third-party certification bodies.
In order to recognize an accreditation body, FDA must review an applicant’s legal authority, competency, capacity, conflict-of-interest safeguards, quality assurance and record procedures. By using an already existing framework familiar to industry, accreditation bodies and certification bodies will be allowed to use documentation of their conformance with the International Organization for Standardization and the International Electrotechnical Commission (ISO/IEC) standards, supplemented if necessary, in meeting program requirements under this rule. An official recognition of an accreditation body is granted for up to five years.
FDA is authorized to recognize a foreign government/agency or a private third party as an accreditation body under TPA.
Recognized accreditation bodies under TPA will be required to:
- Evaluate potential third-party certification bodies for accreditation, including observing representative samples of the prospective certification body’s work
- Monitor performance of the third-party certification bodies it has accredited, including periodical on-site observations, and notifying the FDA of any change in, or withdrawal of, accreditations it has granted
- Self-evaluate and correct any problems in their own performance
- Submit monitoring and self-assessment reports and other notifications to the FDA
- Maintain and provide the FDA access to records required to be kept under the program
Once accredited, third-party certification bodies under TPA are required to perform unannounced facility audits, and to notify the FDA if a condition is found that could cause or contribute to a serious risk to public health.