Compliance to FSMA has presented a new and difficult challenge for industry, the public and the FDA since it passed on January 4, 2011. With compliance dates for the initial FSMA rule—Preventive Controls—coming in September 2016, food sites must establish plans now to meet the impending deadline.
Complying with the Preventive Controls Rule
The Preventive Controls Rule was published September 17, 2015, with the compliance date for registered companies (more than 500 employees) scheduled for September 19, 2016. The compliance date is one year later for companies with fewer than 500 employees, unless otherwise specified under FSMA.
Under the FSMA rules, registered food facilities must evaluate and implement preventive control provisions and meet the requirements and the approaching deadline. The most urgent concerns for companies subject to the Preventive Controls Rule include developing a Preventive Controls Program, identifying a Preventive Control Qualified Individual (PCQI), and implementing a Food Safety Plan.
The following areas are all included under the FSMA Preventive Controls Rule:
- Hazard Analysis. Companies must identify and evaluate known and reasonably foreseeable hazards.
- Preventive Controls. Preventive controls must be implemented to significantly minimize or prevent the occurrence of hazards.
- Monitoring. Preventive controls must be monitored for effectiveness.
- Corrective Actions. Procedures for addressing failures of preventive controls and prevention of affected food from entering commerce are required.
- Verification. Facilities are required to verify that preventive controls, monitoring and corrective actions are adequate.
- Recordkeeping. Records must be kept for two years.
- Written Plan and Documentation. A written plan must document and describe procedures used to comply with requirements.
- Qualified Individual. A Qualified Individual who has been adequately trained must be present at the facility to manage the preventive controls for the site and the products processed and distributed at/from the site.
Failure to implement Preventive Controls (a.k.a., Hazard Analysis and Risk-based Preventive Controls (HARPC)) for qualified sites may result in fines and possible jail sentences.
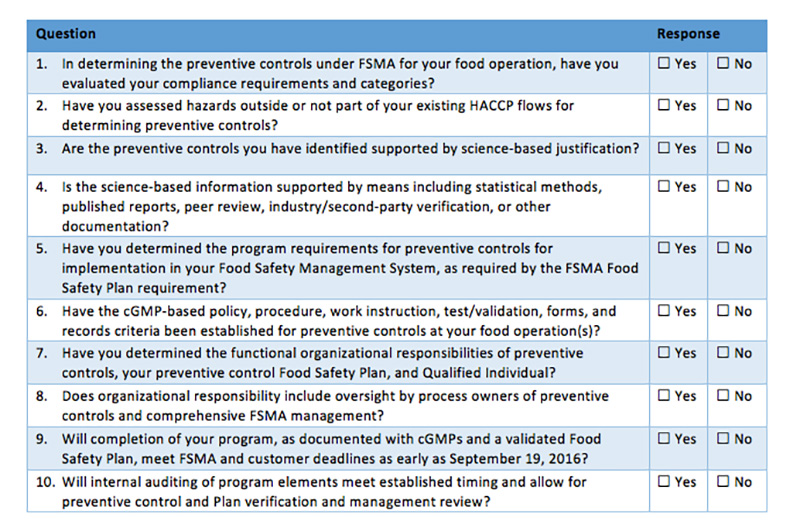
Self-Diagnostic Assessment Tool
The following self-diagnostic assessment tool can help organizations better determine their current state of planning for FSMA compliance (see Table I). To complete your own planning assessment, review your progress compared to the questions below.
Get Compliance-Ready
Companies must have their training, planning and development underway to comply, or face possible violations, fines, and penalties under FDA enforcement. The questions in Table I will help companies identify the areas in which they need to focus attention. Kestrel can also help answer questions, provide input on solutions, discuss how to better manage the preventive controls program—and change “No” responses into “Yes” responses that promote best practices for FSMA compliance.






