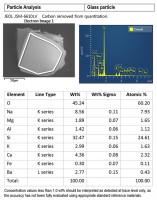
Across the board, increased employee awareness and training has become a big issue in food safety. The foodborne illness outbreaks that hit Chipotle Mexican Grill has put retail and restaurant establishments on high alert, yet this is just another example of the reactive culture in which we operate, according to Matt Schiering, vice president and general manager at Sani Professional.

Food Safety Tech: How is Sani Professional raising the level awareness of the disadvantages of the traditional cleaning method (the rag and bucket method) in the retail environment?
Matt Schiering: There are a few ways to raise the level of awareness. The first and foremost is “feet on the street”. We’ve deliberately moved toward a direct-to-customer sales force, which gives us the opportunity to interface directly with QA, food safety and operations to show them a simpler, more efficient, more effective, and guest appealing way versus the traditional rag and bucket. The first win is one for the user (the employees of a given establishment), because associates have shown us time and time again that they do not like the mixing and measuring, and the errors that are often associated with that process. They don’t like the dirty rag itself—having to fish it out of the bowl and then present it or be seen with it in the front of the establishment. It’s a win for the operator (the manager), because with our system, there’s no longer any heightened heart rate when the health inspector shows up. One of the most common violations is the water in the buckets being out of spec or the rags themselves not being inside the bucket per regulation. And perhaps most importantly, it’s a win for the guest. Think about your own restaurant experiences. Guests don’t want to see or be confronted with a greyish brown rag [that is used to] wipe a table, then wipe a seat, then wipe an adjacent table. It just screams unclean.
As we talk about the evolution in perception, away from traditional methods, we believe that speaking directly to the consumer has to play a role. There has to be a degree of consumer-driven advocacy for a better way. – Matt Schiering
FST: Regarding employee training, how should retailers be more proactive in ensuring their employees are engaging in proper food safety practices and aren’t spreading foodborne illnesses?
Schiering: It varies by chain. Unfortunately, we live in a reactive culture—and that goes well beyond the restaurant industry.
Oftentimes a problem precedes a protocol or other means of addressing said problem. Chipotle is one example: They’ve taken an exhaustive look at restructuring their food safety protocols as a result of a myriad of foodborne illness-related issues that they suffered in the preceding months. The [retailers] who are doing it best are the ones who build it into their establishment in the first place where it’s not predicated by some sort of problem. That involves training materials, in-service lessons, and online training (i.e., ServSafe certifications). Waffle House, for example, has Waffle House University where food safety is a key component to that system.
We envision ourselves as part of that process. We take a microcosm—the notion of proper food handling, prevention of cross contamination related foodborne illness—and provide an innovative and easy-to-use solution, and all the training and collateral materials associated with the solution that explain the proper use. We also provide test kits so that if the health inspector wants an in-the-moment proof that our product is doing what the label says it does, [the retailer] can provide that at a moments notice. It becomes more of a service proposition than simply a product-driven solution.

FST: Where do you see sustainable products fitting into the space?
Schiering: This also boils down to education, because the perception of disposables is that they’re wasteful, when in fact they needn’t be any more costly than existing solutions.
If you’re using a linen service, there’s a cost associated with renting towels, but there’s a higher cost associated with wasting towels. So if a towel ends up in a gym bag or in the trash because of overuse and/or abuse, there’s a significant upcharge for not returning that towel to the rental agency. That’s what we call the hidden cost or the dirty little secret of rag and bucket sanitizing. When you factor that in, and everyone [retailers] experiences that type of loss, and you look at the fact that sanitizing wipes kill pathogens trapped in the wipe as well as whatever it is coming into contact with at the surface, thereby enabling it to be used on multiple surfaces without causing cross contamination—the cost aligns very closely. And of course it’s a more value-added guest experience than a dirty rag being used from table to table, which is not preventing cross contamination.
Speaking to the environmental piece: At the moment, we’re actually fairly well ahead of the industry. It varies chain to chain—some chains are doing a better job than others, because it’s part of their corporate culture. But by providing solutions that are leveraging either recyclable substrates or compostable substrates, we provide greater opportunity to reduce the environmental impact often associated with disposable products. If a retailer is working with a waste management partner that can handle industrial compostable products or non-solid state recyclables, we have solutions that are appropriate for those operations, so that we’re not just adding to landfills but rather essentially recycling and/or regenerating the products that are being used, and at no greater cost.
Most retailers haven’t gotten there yet. It speaks directly to corporate culture and corporate mission of the end user. We deliberately target customers who are a little bit ahead of the curve when it comes to “green technology or “green behavior”. And so when the rest of the industry catches up, we’re more than ready to serve them with products that meet those needs.
FST: Where do consumers fit into the picture, especially has industry moves away from traditional methods in food safety?
Schiering: About a decade ago, consumers started demanding that retailers like Walmart, Target, and local grocers provide a means of sanitizing shopping carts when they walk into their local retail establishments. There were myriad news reports about the germs and potential for contamination and illness arising from the often used and rarely cleaned implements—these vehicles for placing your groceries. We answered the call a decade ago, and at one time it was a significant piece of our business. It continues to be a marketplace we serve, albeit a much commoditized one. But the rise in that solution would not have taken place if not for consumers advocating for a better way.
We’re starting to create a presence on Facebook and other social media outlets to remind consumers that it’s up to them in many cases to ask for, if not demand a more effective, more pleasing way of ensuring their safety in dining establishments. Unfortunately, incidents like what we saw at the large Mexican food service retailer do ultimately play a part in that consumer advocacy, albeit a negative one, because we are a reactive society. But by presenting a positive message and sharing alternatives in the absence of citing examples or shaming retailers through the problem, we believe that will be one of the keys to changing perceptions at the retail level.