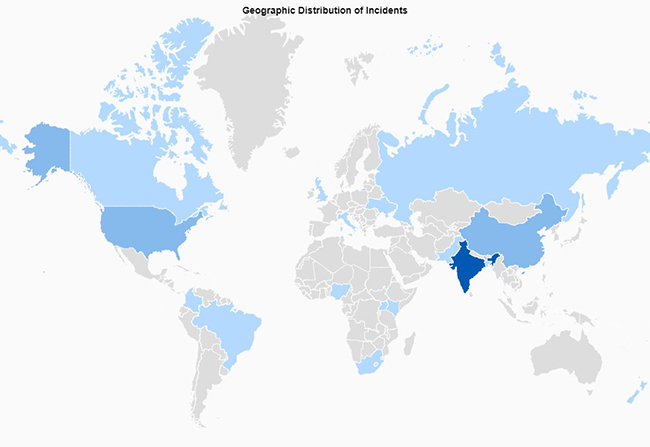
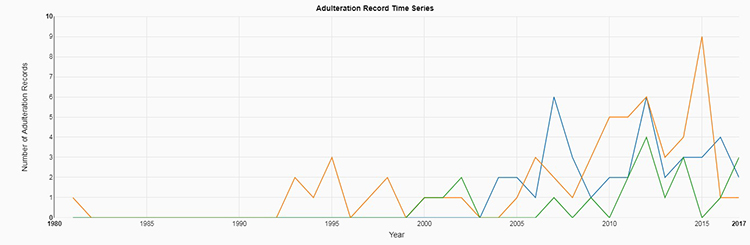
Everybody knows to prepare for the worst while hoping for the best. But being prepared for an unannounced audit isn’t something that can be done overnight, especially when it comes to pest management.
That’s why it’s important to carefully review your food safety plan and the specific pest management measures contained within it. Pest management can account for up to 20% of an audit, so it can make or break a facility’s score. There’s no room to gamble—between federal regulations, audit regulations, internal standards and customer expectations, you need to stay in compliance.
With FSMA in full effect, it’s important to become familiar with the regulations found on the FDA’s website if you haven’t yet. Reacting to problems isn’t good enough anymore, as many of these regulations emphasize a preventive strategy. The FDA has the authority to mandate recalls, shut down facilities and more. If you need help determining the rest of your facility’s food safety program, the FDA has a helpful downloadable food safety plan builder.
To make sure pest issues don’t spoil your score during an unannounced audit, remember that pest management is an ongoing partnership that requires your entire staff’s participation. It’s something you should plan for as part of regular maintenance and sanitation schedules.
The first step is to establish an integrated pest management (IPM) program. This plan should be developed with your pest management provider and should be a comprehensive look at all factors that may contribute to pest issues. It should be designed to reduce pest pressure around a facility, address all sanitation and exclusion issues, actively monitor for pests and set action levels.
The goal is to proactively deal with pests and prevent as many issues as possible. An IPM program is tailored to meet a facility’s specific needs based on a wide range of different factors. An IPM program uses pesticide treatments as a final step once all conducive conditions have been identified and addressed. The program should adapt as both the facility and the pest pressures change over time.
Implement regular training for all employees and encourage participation. Something as simple as a poster featuring the “most wanted pests” in break rooms or locker rooms can help train personnel to recognize the signs of potential pests around the facility. Make it clear that each employee is a key player in your program and empower them to speak up should they see something. Ensure you have a pest-sighting log and all personnel are aware of where it is and how to report issues. Their help will be vital to point out issues, ensure sanitation is adequate and identify problem areas on a daily basis. Your pest control professional can host a training meeting for you and your staff if needed.
When implementing an IPM program at your facility, there are numerous preventive tactics you can implement and perform on a regular basis.
- Monitoring devices: Using devices like fly lights, rodent traps and bait stations can help reduce pest populations and keep them away from food products. These monitoring devices also offer insight as to how many and what kinds of pests are plaguing the facility, which is valuable information when determining a strategy to resolve existing pest problems and minimize pest pressure.
- Sanitation: Any food source, including the foods you are producing and storing, can draw pests. Clean up product spills quickly. Don’t forget about food in employee areas such as break rooms and locker rooms. While it is impossible to clean up every food particle (you are producing food at your site!), you can work to limit the access pests have to food sources.
- Take out the trash: Emptying trash daily and cleaning out the trash bins helps prevent the buildup of organic material, which can attract many different pests. Make sure dumpsters are placed away from the building if possible and always keep them closed. Don’t a smelly garbage or a full bin—when in doubt, take it out!
- Seal cracks and crevices: Keep a constant watch around the facility for any openings big enough for a pest to fit through. Remind employees that rats only need a quarter-size hole to squeeze into a facility, while mice only need an opening the size of a dime. And that’s not the worst of it; cockroaches only need one sixteenth of an inch, making it vital to seal off any openings found. Don’t forget to look at your facility from the outside as well!
- Install automatic doors: Often, open doorways are prime locations for pests to enter. All doors should remain closed when not in use. Especially in docking areas when loading/unloading, try to open dock doors only when needed and keep the gap from the truck to doorway sealed off if possible. Installing automatic doors can reduce the likelihood flying pests inside the facility by ensuring doors stay closed when not in use.
- Inspect shipments: Anytime a new shipment arrives, it should be inspected closely for pests. Stored product pests could be in products and spread to others in close proximity quickly, so catching them early is key. Don’t forget to inspect shipping containers for outgoing product too, because if your product goes in an infested truck, your product will be considered infested!
- Remove clutter: Many pests love to hide under clutter. Remove unused equipment, product and packaging—especially cardboard boxes—to avoid giving pests a convenient hiding place. Don’t forget about clutter outdoors, too.
- Outdoor concerns: Sanitation is important outside the facility as well. Make sure to remove clutter and garbage on the ground and near the facility to help reduce pest pressure. Many pest issues start outside, so removing clutter outdoors means fewer pests to potentially get into your site. In addition, installing sodium vapor lights instead of mercury vapor lights near the building will attract fewer insects.
- Install air curtains: The perfect complement to automatic doors, air curtains create positive airflow—or air flowing outwards from building entrances—to push pests away from the building.
Auditors are looking for a number of things when it comes to pest management. One important item they want to see is the record of past pest issues and the steps taken to resolve them. You and your pest management provider should ensure all records are up to date and accurate with pest trends that can be explained.
Documentation is perhaps the most critical part of a strong IPM program. It ensures your efforts are captured, organized and available should an unexpected auditor arrive on site.
The following are six main documents to have ready at all times.
- Food Safety Plan
Perhaps the most important piece of documentation, the overarching food safety plan is absolutely necessary to have on hand. The plan should be a comprehensive document describing all activities to ensure the safety of food during manufacturing, processing, packing and holding. It should include a list of potential hazards, preventive controls and corrective actions to mitigate those risks, along with monitoring and verification procedures. - List of Service Changes
An IPM program needs to be dynamic. But when modifications are made to meet the ever-changing needs of a facility, make sure to keep careful records of how and why the plans have changed. - List of Monitoring Devices/Traps
Your plan must include a map documenting all monitoring equipment, traps and any other devices used around the facility to reduce pest pressure. Note the locations and activity levels of each. The trend report from the collected data can show important information and help make management decisions. Your pest management professionals can help with this, as they should be noting activity each time they inspect your property. Auditors will want to see the historical data of pest monitoring devices and the corrective actions associated with any issues. Monitoring devices work as a great early warning system for developing pest issues and are a great proactive approach. - Annual Assessments
Each year, you should review your food safety plan and current IPM program. These annual assessments will note problem areas and set goals for the coming year. Auditors will be looking for these yearly assessments, and if you’re able to demonstrate year-over-year improvement then you’ll give your facility a better chance at a great audit score. - Sighting Reports
If a pest is spotted within the facility, employees should document it on the pest-sighting log. The report should include information about the location of the pest within the facility, who found it and the number of pests spotted. Capturing the pest is ideal, but it’s not always feasible to do so. In that case, photo evidence helps with identification, so obtain a close-up picture of the pest(s) if possible. Ensure the pest is identified and any corrective actions documented. - Proof of Training/Certification
You know that your pest management professional is trained and certified, but an auditor doesn’t. To demonstrate your provider’s expertise, keep a valid license or certification document, written evidence of the pest management professional’s training, and documentation of internal training on IPM and Good Manufacturing Practices (GMPs).
According to FSMA guidelines, a strong food safety plan identifies potential hazards to food products, and focuses on a preventive, risk-based strategy instead of a reactionary one. With an IPM program in place and detailed documentation of actions taken, you’ll be prepared anytime an auditor decides to “pop in” for a “quick chat.”