In the following article, the author reports finding Sudan dye in spices in New York State, making the argument for Class I recalls.
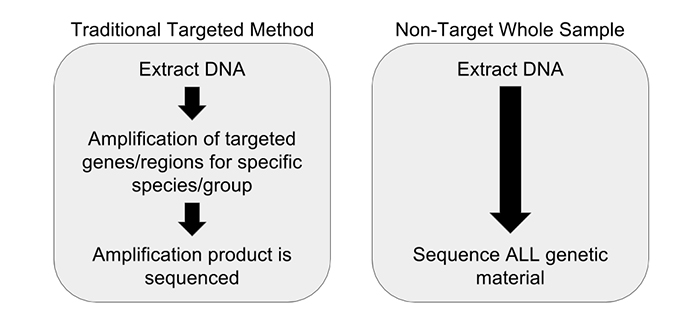
In New York State (NYS), Department of Agriculture and Markets food inspectors routinely sample domestic and imported food from retail markets for food dye determination. For decades, the NYS Food Lab has examined both domestic and imported food for undeclared allowed food dyes and unallowed food dyes utilizing a paper chromatography method. This method works well with water-soluble acid dyes, of which food dyes are a subset.
The NYS Food Lab has participated in four sets of the FAPAS proficiency tests: Artificial Colours in Soft Drinks and Artificial Colours in Sugar Confectionary (Boiled Sweets). The qualitative analysis was by paper, thin layer silica and thin layer cellulose chromatography. Satisfactory results were obtained.
The paper/thin layer chromatography method is a qualitative non-targeted method and has a limit of detection of approximately 1 to 5 ppm (parts per million) depending on the dye. If an unallowed dye is detected, the food product is violated as adulterated and results are forwarded to the FDA.
Some countries have a maximum concentration of allowed food dye in a food product. For example India has a 100 ppm to 200 ppm maximum for their allowed food dyes, in some food, singly or in combination.1

In early 2011, a food sample of pink colored sugar coated sesame seed from Pakistan was sent to the lab for color determination. The paper chromatography method could not determine any dyes. (As found out later, the unknown pink dye was not an acid dye.) From research it was found that Rhodamine B was a pink water soluble basic dye commonly used as a food adulterant. A standard was ordered and then a qualitative high performance Liquid chromatography-tandem mass spectrometry (HPLC/MS/MS) method was developed (Waters UPLC Aquity w/Waters Premier XE triple quadrapole) to determine Rhodamine B. After utilizing this new method, Rhodamine B was found in the sugar coated sesame seed.
Rhodamine B is an industrial dye and is not allowed in food anywhere in the world. Industrial dyes are not allowed in food because they are toxic; in fact, some industrial dyes are used for suicide.2,3,4 In addition, industrial dyes are not made to “food grade” specifications with regard to dye purity, heavy metal (i.e., arsenic and lead) concentrations, subsidiary dye concentrations and concentrations of unreacted precursors. From additional research of news articles and research papers, more industrial dyes were identified as common food adulterants; more dye standards were ordered and incorporated into the HPLC/MS/MS method. The NYS Food Lab’s current HPLC/MS/MS surveillance method includes 36 compounds: Water soluble “acid dyes” and “basic dyes”, organic solvent soluble “solvent dyes”, and several pigments.
The HPLC/MS/MS method has a limit of detection in the ppb (parts per billion) range for some dyes and parts per trillion for other dyes. The FDA has an action level of 1 ppb for certain water-soluble basic dyes (such as Malachite Green) when used as a fish antibiotic. However, due to concern that unallowed dyes might be present due to contamination from packaging, the food lab subsequently set an action level of 1 ppm for unallowed dyes determined by the HPLC/MS/MS method. At levels over 1 ppm, detection of dyes in food would indicate intentional dye usage for coloring food.
The food lab has participated in three rounds of the FAPAS proficiency test, “Illegal Dyes found in Hot Pepper Sauce”. The qualitative analysis was by LC/MS/MS. Satisfactory results were obtained.
Sudan Dyes Considered to be Carcinogenic
“Sudan dyes are not allowed to be added to food. There has been worldwide concern about the contamination of chili powder, other spices, and baked foods with Sudan dyes since they may have genotoxic and carcinogenic effects (according to the International Agency for Research on Cancer)”.5
“There have been several documented cases of spices being contaminated with carcinogenic dyes such as Sudan I or lead oxide. We therefore assume that the presence of these chemicals in spice ingredients will be considered a reasonably foreseeable hazard under this rule.”6
“Sudan red dyes have been used to color paprika, chili powders, and curries, but are also known carcinogens and are banned for use in foods.” 7
Sudan Dyes are a family of more than 10 synthetic industrial “solvent dyes”. Solvent dyes are typically used to color oils and waxes, including shoe polish. Sudan dyes that the food lab has found in spices include Sudan 1 (Sudan I), and Sudan 4 (Sudan IV). Sudan 1, also known as Solvent Yellow 14, is an orange colored dye. Sudan 4, also known as Solvent Red 24, is a blue shade red colored dye.
Positive identification of Sudan 4 is often hindered by the existence of a positional isomer, Sudan Red B (Solvent Red 25). This problem was addressed by using the HPLC/MS/MS method with a transition unique to Sudan 4 (381.2 > 276.0). This information was obtained from one of the two corroborating labs. The food lab has recently identified a transition unique to Sudan Red B (381.2 > 366.1).
Sudan Dyes Found in Spices in Europe
In March 2001, Europe began discovering Sudan dyes in spices. A February 2017 search of Europe’s Rapid Alert System for Food and Feed (RASFF) for “unauthorised colour” and “sudan” in the “herbs and spices” food category resulted in 429 notifications.
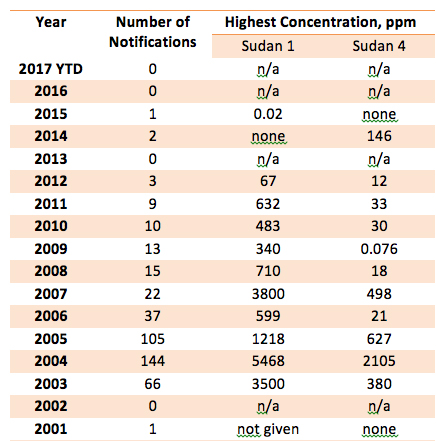
The 429 RASFF notifications arranged by year and by maximum concentration reported of Sudan 1 and Sudan 4 during that year are listed in Table I.
In a search of the FDA’s Import Alert 45-02 (Detention Without Physical Examination and Guidance of Foods Containing Illegal and/or Undeclared Colors) the author could find no record of spices violated for Sudan dye adulteration.
In a search of the FDA’s Enforcement Reports the author could find no record of spices violated for Sudan dye adulteration.
Industrial Dyes in Food: Class II or Class I Recall?
The NYS Food Lab and the FDA routinely find imported food containing unallowed food dyes such as Ponceau 4R, Amaranth and Carmoisine. These unallowed food dyes are allowed for use in food in other parts of the world, while not allowed in the USA. Foods containing unallowed food dyes are violated as adulterated and a Class II recall will occur. Sudan dyes are not allowed as food dyes anywhere in the world. They are industrial dyes, used in coloring oils and waxes, such as shoe polish.
“Class I recall: A situation in which there is a reasonable probability that the use of or exposure to a violative product will cause serious adverse health consequences or death.
Class II recall: A situation in which use of or exposure to a violative product may cause temporary or medically reversible adverse health consequences or where the probability of serious adverse health consequences is remote.”8
With a Class II recall, there is no consumer notification. In contrast, as part of a Class I recall, a press release is issued. Consumers who have purchased the product might be informed and may discard the product or return it for a refund.
Continue to page 2 below.