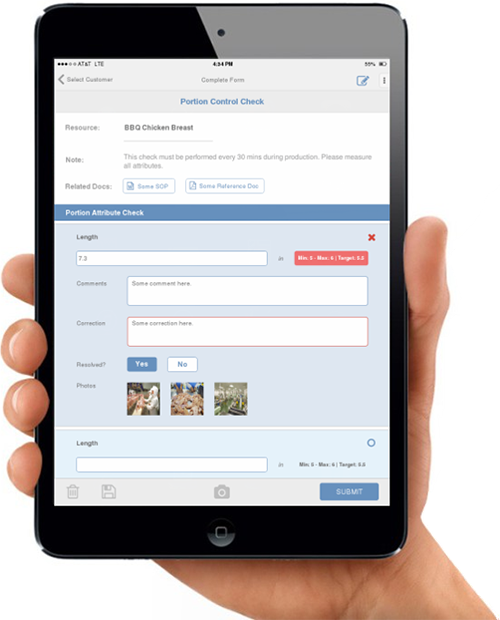
As the popular phrase goes, if you’re going to talk the talk, then you need to walk the walk. This expression really does ring true when discussing an effective food safety culture within an organization. Timothy Ahn, senior technical manager of food safety at LRQA sheds some light on the importance of management commitment as a foundation for success in implementing a food safety culture and how employee training fits into the picture.
Food Safety Tech: In your column on Food Safety Tech, “Tackling the ‘Why’ of Food Safety”, you touch on the point that food safety culture needs to start at the top. What are the issues in management today that prevents a food safety culture from flourishing within organizations?
Timothy Ahn: First, it’s important to define food safety culture. It can mean a lot of different things to people. The culture is the collective behavior from the organization around shared values and beliefs. From that perspective, it’s extremely important the leadership understands its beliefs and values. The collective behaviors of the leaders become really important, because we’re also talking about how the leadership sets management commitment and drives what’s important in the organization. The organization will follow whatever the leaders do and not necessarily what they say. That’s the issue—having the ability to get commitment from leadership that is demonstrated through their actions, which then transfers into rewards, objectives and consequences. What are the issues that are preventing the culture from embedding itself? Actions aren’t aligned with their words. You have a senior leadership group that will say one thing, but then their actions are different.
In addition, when cascading priorities are very different, food safety doesn’t get the right messages. It’s about growth, market share, profits—all of those financial measures are extremely important, because they have consequences and are also rewarded. Meanwhile, depending on the organization, the objectives around food safety culture may or may not be talked about, defined, or even rewarded. It’s really about making sure that the organization has cascading priorities.
FST: When taking a holistic approach to employee training, what are some of the challenges that companies can expect to encounter?
Ahn: I put food safety into three different buckets that build on top of each other.
- At the bottom is the foundation. It’s around good manufacturing practices and all the foundational activities that need to be in place for factory operations. How often you clean your equipment? What do you do around allergens? Can you trace your materials from one end to the other? Do you have a pest control program?
- Our food safety system: This includes things around HACCP: Do you have a HACCP plan in place? Do you understand what your hazards are? Have you defined your control measures?
- The last bucket is around the management system that drives food safety. Have you defined your objectives? Do you have a policy? Do you conduct a management review? Do you have an internal audit?
The issue with training is many operations only focus on the foundation. You need to have people who know how to clean equipment; you have to make sure that the pest control is done; you need to have good allergen management. Those are all pretty well done. Now you’re starting to get more traction in the second bucket, which is around HACCP, because with FSMA, HACCP is no longer an option; you need to be able to do it.
But the missing piece in many organizations is at the top—the management systems. This is important, because when you talk about culture, that’s where it gets embedded within an organization—through implementation of the management system.
If you look at this holistically, you need to train across all of those areas, not just in the foundation. You can differentiate yourself from organizations that have effective food safety management systems (not just food safety systems) because they’re training across all those buckets.
The other part of training is management systems. Who do you train? Besides targeting first line employees and operators, you also need to train senior managers because these managers, along with leadership, need to better define the objectives and policies. What does it mean to conduct an effective management review? What does it mean to do an internal audit? What’s a good corrective action process? The training often falls apart because organizations haven’t embedded that very well.
FST: Do you see differences between implementing these practices in small versus large organizations?
Ahn: There are differences, but it’s not necessarily a function of the size of the company. It’s more around how they’ve approached developing and organizing their management system and in particular, their food safety management system.
FST: In reality, how long does it take a typical company to create an effective food safety culture?
Ahn: There are two parts to that question.
My belief is that if you want to implement a food safety culture, you need to create a food safety management system, otherwise it is just all words and talk. It’s what you do, not what you say. The way to do that is to embed a food safety management system within your organization.
The two questions are: How do you get to initiate it? And, how long does it take to execute once you decide to initiate?
To address the first question: How do you initiate it?
There are a couple of ways that it can happen. There’s nothing like a crisis to get the fire under somebody’s feet, whether it’s a recall or an incident, it will draw attention to the fact that there’s a problem and something needs to happen. But, that’s reactive and detrimental.
The other way to initiate is that if you have enlightened leadership—an owner or group of owners who understand where they want to go, where they need to go, what needs to be avoided and understands the importance of the organization’s culture in getting to the right place, etc.
Secondly, once you start this process, how long does it take to get this type of system running?
Based on my experience in implementing food safety management systems like FSSC 22000, it takes anywhere from 18 months to 2 years to get it established, and then probably another 18 months or so to actually fully implement. So it’s not something that happens in a couple of months. It takes some time to really get it implemented and embedded, because these are foundational elements you must put into place. There’s a lot of momentum involved, and it has to move throughout the organization.
The term food safety culture has gotten a lot of attention—it’s a buzzword. But what does it really mean and how do you make it come to life? That’s really where people need to start looking. You make it come to life through implementation of structured food safety management systems—ones that are verified, and independently verified. Put substance and real work around your food safety culture instead of using a lot of fluffy words to describe it.